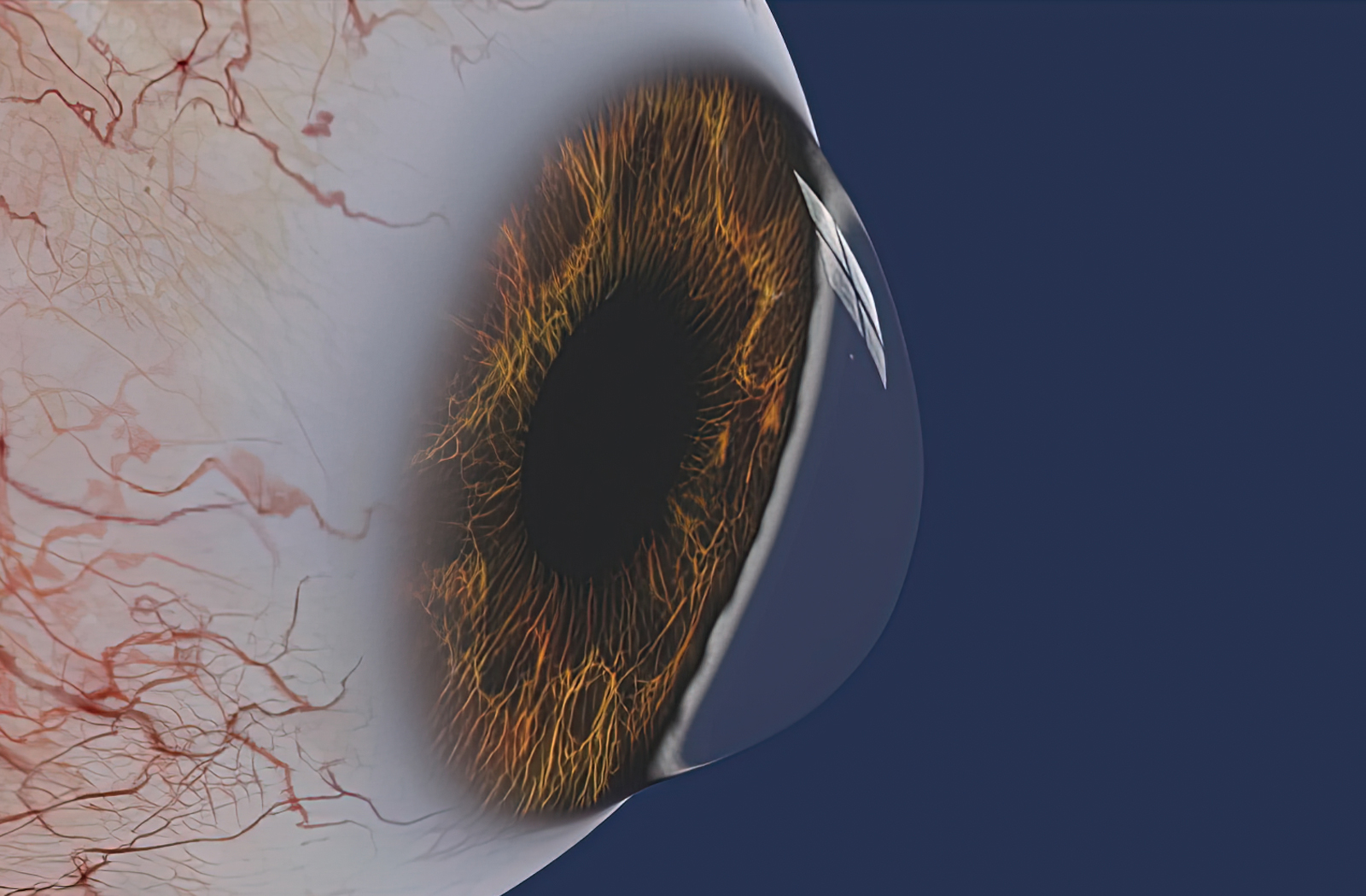

Cross-Linking Treatment for Keratoconus or Corneal Ectasia
A minimally invasive method for the treatment of Keratoconus
Cross-linking is an alternative to corneal transplantation to retain vision. If diagnosed with progressive keratoconus, FDA-approved cross-linking is a treatment option that is clinically proven to limit the progression of the sight-threatening disease.
Cross-linking was pioneered in 1997 and eventually approved by the FDA in 2016. It is an office-based procedure that can slow, stop, and often reverse the effects of Keratoconus and post-LASIK ectasia. Patients should expect their corrected and uncorrected vision to improve and their risk of requiring corneal transplantation to decrease. As one of the original Avedro FDA investigation sites, our office performs cross-linking in a sterile, climate controlled operating suite using the latest technology.
Most major insurance companies cover cross-linking. For patients without adequate health policies we employ special programs and secure industry support to assure this vision preserving technology is available in all appropriate cases.

